

TheclassicMynox®isanantibiotic-freeformulation;itsactivityisbasedonabiophysicalmechanism,thusthereisnodevelopmentofresistantstrains.Mycoplasmaarepermanentlydestroyedwithin2-3hours.
Benefits
Freeofantibiotics
RecommendedUse/Scope
Mynox®isintendedforresearchuseonly.Notapplicableforclinicaltreatment.ThedirecttreatmentofenvelopedvirusesisnotpossIBLe.
KitComponents
Mynox®isasterile,ready-to-usesolution,aliquotedpertubeforsingleuse,200µlpervial.
PackageSizes
1applicationcanbeusedtocureonecellculturepermanentlyfrommycoplasmaburden.
- Cat.No.10-02002applications
Cat.No.10-05005applications
Cat.No.10-100010applications
ResultEvaluation
Eliminationsuccessshouldbeverifiedwithasuitablemycoplasmadetectionsystem,e.g.MinervaBiolabs’sVenor®GeMMycoplasmaPCRDetectionKits.
RequiredConsumables
6-welltissuecultureplateorsmalltissueculturepetridish(6mmØ)
Regularcellcultureplastics
RequiredLabDevices
Regularcellcultureequipment(incubator,centrifuge,Pipettes,etc.)
ShelfLifeandStorage
Kitcomponentsaremaintainableat+2to+8°Cforatleast6months.
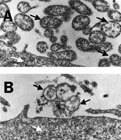
Fig.ElectronmicrographshowingtheeffectsofMynox®whenappliedtocontaminatedcellcultures.(A)Mycoplasmacontaminatedcellculturebeforetreatment.(B)WithoneapplicationofMynox®membranesofallmycoplasmaaredestroyedwhilethetissuecellmembraneisunaffected.Blackarrowsindicatemycoplasmas,whitearrowsindicatepartsofatissuecell.
![]()
暂无问答





























